Cardinal Health launches webpage for gown recall updates
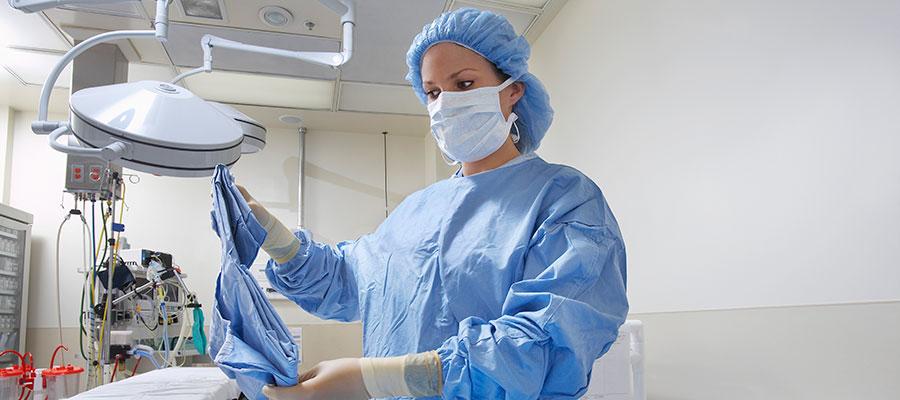
Cardinal Health has created a webpage, www.cardinalhealth.com/SurgicalGownProductRecall, to keep customers informed about its Jan. 21 recall of certain surgical gowns because it could not assure their sterility. The company recalled 9.1 million AAMI Level 3 gowns produced by a former contractor after learning that some of the gowns were made at unapproved locations that did not maintain proper environmental conditions. About 1.4 million of the gowns were not distributed. For more on the recall, see the Jan. 22 AHA Quality Advisory.
Related News Articles
Headline
The measles outbreak in South Carolina has increased to 876 cases, the state’s Department of Public Health reported Feb. 3. Last week, the South Carolina…
Headline
Thomas McGinn, M.D., senior executive vice president and chief physician executive officer at CommonSpirit Health, shares how the organization aligns…
Headline
The Centers for Disease Control and Prevention released its annual progress report on health care-associated infections Jan. 29, which found continued…
Headline
Two AHA guides offer strategies for hospitals and health systems in preparing for public health emergencies and disasters and managing cybersecurity incidents…
Headline
Stephanie Calcasola, R.N., chief quality officer and vice president of quality and safety at Hartford HealthCare, unpacks the programs, technology and cultural…
Headline
Wendy Kim, DNP, R.N., vice president and chief nursing officer of Henry Ford Health in Michigan, shares how the system’s virtual nursing program is reducing…

