Fact Sheet: Extending the Hospital-at-Home Program
December 2025
The Issue
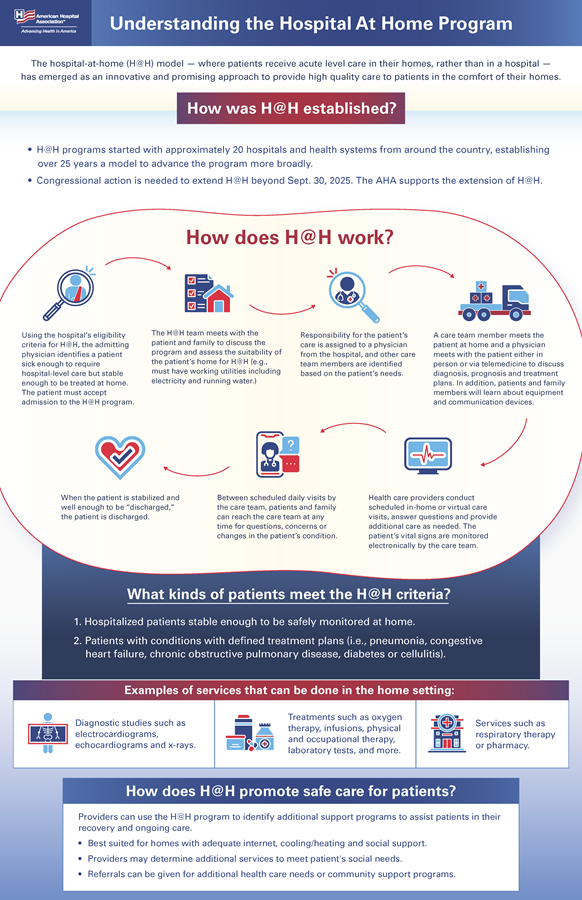
The hospital-at-home (H@H) model — where patients receive acute level care in their homes, rather than in a hospital — has emerged as an innovative and promising approach to provide high quality care to patients in the comfort of their homes. Since the start of the program, Congress has extended it repeatedly for short time periods: first in 2022 (Consolidated Appropriations Act of 2023) for two years, second in 2024 (H.R. 10545, the American Relief Act) for 90 days, third in March 2025 (H.R. 1968, the Full-Year Continuing Appropriations and Extensions Act) for six months and fourth in September 2025 (H.R. 5371, the Continuing Appropriations, Agriculture, Legislative Branch, Military Construction and Veterans Affairs, and Extensions Act, 2026) for 78 days. These extensions received no score from the Congressional Budget Office. Congressional action is needed to extend the waivers for this program, which are now set to expire Jan. 30, 2026.
On Dec. 1, 2025, the House of Representatives voted to pass the Hospital Inpatient Services Modernization Act (H.R. 4313 / S.2237), which would extend hospital-at-home programs through the end of 2030. The bill now moves to the Senate for consideration.
AHA Take
The AHA supports the Hospital Inpatient Services Modernization Act, which was introduced in the House by Reps. Vern Buchanan, R-Fla., Lloyd Smucker, R-Pa., and Dwight Evans, D-Pa., and in the Senate by Sens. Tim Scott, R-S.C., and Rev. Raphael Warnock, D-Ga. The bill extends the H@H waiver for five years and directs the Centers for Medicare & Medicaid Services (CMS) to conduct a new study of the program.
Hospitals and health systems see H@H programs as a safe and innovative way to care for patients in the comfort of their homes. This kind of care is well suited for medium acuity patients who need hospital-level care but are considered stable enough to be safely monitored from home. Rather than staying three days or longer in the hospital, these patients can be treated safely by their doctor and a team of medical professionals along with the patient’s support system at home.
A long-term extension of the H@H waiver will not only provide additional time to continue gathering data on quality improvement, cost savings, and patient experience, but will also provide much-needed stability for new programs and may ease state concerns about updating Medicaid policies to cover these services. The recent 43-day shutdown of the program created significant disruption for patients, providers and administrators, underscoring the urgent need for continuity and reliable support for H@H initiatives.
Background
To allow hospitals and health systems the ability to respond to the COVID-19 pandemic effectively and efficiently, CMS provided a number of waivers and flexibilities that eased several Medicare restrictions and requirements. These included waivers to certain conditions of participation for approved H@H programs.
To receive approval to participate in the H@H program, hospitals must submit an individual waiver request to CMS. The request specifically asks CMS to waive §422.23(b) and (b)(1) of the Medicare Conditions of Participation, which require nursing services to be provided on premises 24 hours a day, seven days a week, as well as the immediate availability of a registered nurse for the care of any patient. Once the waiver request is received, CMS divides the applications into two categories, allowing more-experienced hospitals a quicker approval process so they can rapidly expand their H@H program; less-experienced hospitals must demonstrate they can meet the requirements associated with the provision of H@H services.
As of September 2025, 419 hospitals across 147 systems and 39 states have been approved to provide H@H services to patients. Other health systems and hospitals have indicated they are interested in standing up H@H programs but are hesitant to do so without a long-term extension from Congress.
In October 2024, CMS released a report adding to the growing body of literature demonstrating that H@H is a safe, effective program. CMS found that H@H patients generally had lower mortality rates, readmission rates and spending in the 30 days post-discharge. Patients and caregivers also expressed predominantly positive experiences with the program. While the report found that H@H patients were more likely to be white and live in an urban location and less likely to receive Medicaid or low-income subsidies, this can in part be attributed to the variability in state Medicaid coverage of H@H programs. As of June 2024, only 12 states provide Medicaid coverage for H@H.