FDA approves first RSV vaccine, for adults 60 and older
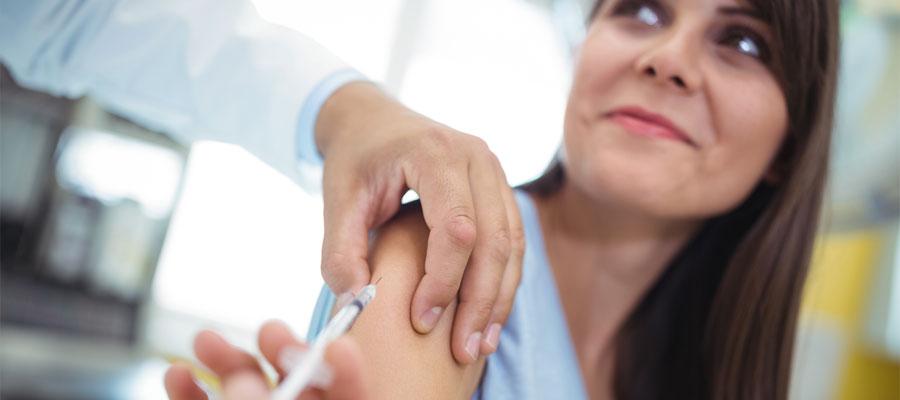
The Food and Drug Administration on May 3 approved the first U.S. vaccine for respiratory syncytial virus, Arexvy by GlaxoSmithKline Biologicals, for use in individuals aged 60 and older.
“Older adults, in particular those with underlying health conditions, such as heart or lung disease or weakened immune systems, are at high risk for severe disease caused by RSV,” said Peter Marks, M.D., director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States.”
Related News Articles
Headline
The AHA today submitted a letter to the Office of Science and Technology Policy in response to its request for information on regulatory reform for artificial…
Headline
The AHA and Federation of American Hospitals Aug. 8 filed an amicus brief in the U.S. District Court for the Eastern District of Texas in support of the U.S.…
Headline
President Trump Aug. 7 issued an executive order, “Improving Oversight of Federal Grantmaking,” requiring government agencies to review new and discretionary…
Headline
The Centers for Medicare & Medicaid Services July 15 issued a proposed rule that would increase Medicare hospital outpatient prospective payment system…
Headline
AHA May 23 submitted recommendations to the Department of Justice and Federal Trade Commission in response to the agencies’ requests for information on…
Headline
The Department of Health and Human Services May 13 announced a 60-day public comment period opened for stakeholders regarding its request for information to…

