FDA evaluating certain plastic syringes made in China
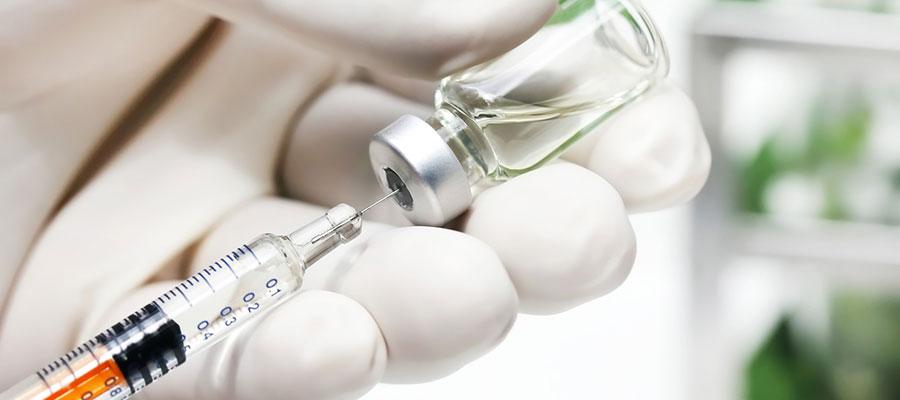
The Food and Drug Administration is evaluating Chinese-made plastic syringes used to inject or withdraw fluids from the body, citing concern that they may not provide adequate quality and performance, including their ability to deliver the correct dose of medication when used alone or with other medical devices, such as infusion pumps. Meanwhile, health care facilities should use other plastic syringes or closely monitor their use, the agency said. The concern does not currently apply to glass syringes, pre-filled syringes, or syringes used for oral or topical purposes, FDA said.
Related News Articles
Headline
The Food and Drug Administration has identified a recall by Cook Medical of Zenith Alpha 2 Thoracic Endovascular Graft proximal components after Cook Medical…
Headline
The Food and Drug Administration has issued early alerts for certain Spectrum infusion pumps from Baxter and Abiomed Automated Impella Controllers. The agency…
Headline
The Centers for Medicare & Medicaid Services Feb. 14 announced it ceased implementation of the Hospice Special Focus Program so the agency can further…
Headline
The White House Feb. 1 announced it placed tariffs on imported goods from Canada, Mexico and China. The tariffs for Mexico and Canada have since been delayed…
Headline
The latest revisions to the Centers for Medicare & Medicaid Services’ Quality Assurance and Performance Improvement program underscore the integral role of…
Headline
Draeger Medical, Inc. is recalling its Carina Ventilators due to the presence of contaminants in the device’s airpath, which exceed acceptable levels if…

