Cardinal Health initiates recall, correction of surgical procedure packs
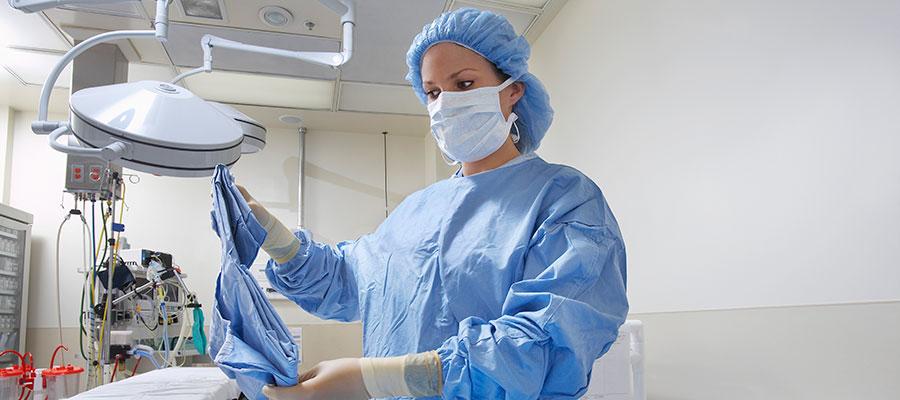
Cardinal Health, in coordination with the Food and Drug Administration, today announced a recall of 2.5 million procedure packs manufactured between September 2018 and January 2020 that contain surgical gowns recalled last week. The company said it also will correct another 374,794 procedure packs with components separated from a recalled gown by inner sealed packaging or other packs within the sterilization pouch. The procedure packs were previously on hold. Cardinal said customers will receive detailed instructions for handling the affected procedure packs on or around Feb. 3. For the latest updates on the issue, visit www.cardinalhealth.com and www.aha.org.
Related News Articles
Headline
The Food and Drug Administration has identified a Class I recall of certain FreeStyle Libre 3 and FreeStyle Libre 3 Plus…
Headline
Thomas McGinn, M.D., senior executive vice president and chief physician executive officer at CommonSpirit Health, shares how the organization aligns…
Headline
The Food and Drug Administration Feb. 3 released an early alert on a heart pump issue from certain Abiomed products. The agency said Abiomed found its Impella…
Headline
Stephanie Calcasola, R.N., chief quality officer and vice president of quality and safety at Hartford HealthCare, unpacks the programs, technology and cultural…
Headline
Wendy Kim, DNP, R.N., vice president and chief nursing officer of Henry Ford Health in Michigan, shares how the system’s virtual nursing program is reducing…
Headline
The Food and Drug Administration has identified a recall by Cook Medical of Zenith Alpha 2 Thoracic Endovascular Graft proximal components after Cook Medical…

