FDA advisory panel recommends monovalent XBB COVID-19 vaccine for fall
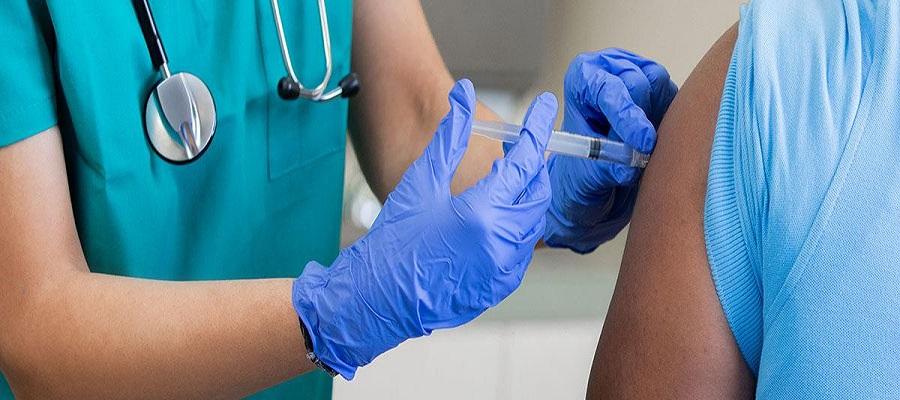
The Food and Drug Administration’s vaccine advisory committee June 15 voted unanimously to recommend updating the current COVID-19 vaccine composition for fall to a monovalent vaccine based on an XBB variant of the virus, the lineage currently predominating in the U.S. The FDA will now consider the recommendation.
Related News Articles
Headline
The Food and Drug Administration today released two guidance documents; one related to low-risk wellness products (including certain wearable devices) and the…
Headline
The Centers for Disease Control and Prevention Dec. 11 released a report that found last year’s version of the COVID-19 vaccine was 76% effective in preventing…
Headline
The AHA provided recommendations to the Food and Drug Administration Dec. 1 in response to a request for information on the measurement and evaluation of…
Headline
The Food and Drug Administration has identified a Class I recall of Baxter Life2000 Ventilation Systems due to a cybersecurity issue discovered through…
Headline
The Food and Drug Administration yesterday published an announcement from Otsuka ICU Medical saying that the company issued a voluntary recall for a mislabeled…
Headline
The Centers for Disease Control and Prevention will update its immunization schedules for the COVID-19 and chickenpox vaccines to adopt recent recommendations…

