Pfizer: COVID-19 vaccine still 100% effective in adolescents after four months
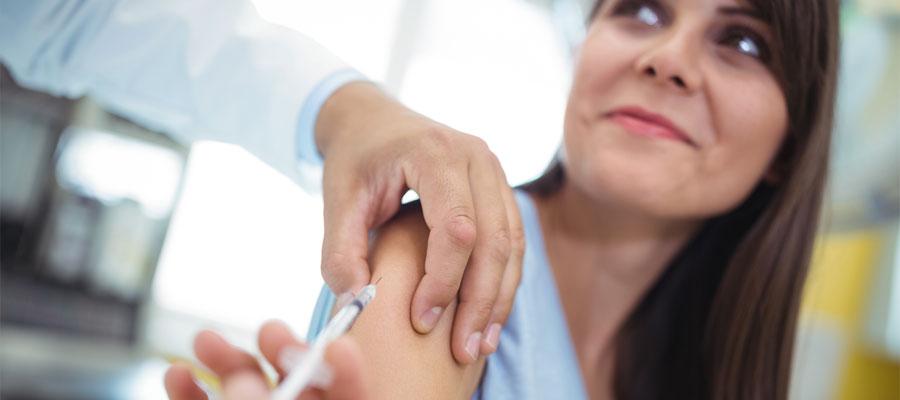
A two-dose series of the Pfizer-BioNTech COVID-19 vaccine was 100% effective against COVID-19 in 12-15 year olds more than four months after the second dose in the companies’ phase 3 clinical trial, Pfizer and BioNTech announced yesterday. The adverse event profile was generally consistent with other clinical safety data for the vaccine, with no serious safety concerns observed in individuals with at least six months of safety follow-up after the second dose, the companies said. They plan to submit the updated data to the Food and Drug Administration, which in May expanded the vaccine’s emergency use authorization to include 12-15 year olds.
Related News Articles
Headline
The Food and Drug Administration today released two guidance documents; one related to low-risk wellness products (including certain wearable devices) and the…
Headline
The Centers for Disease Control and Prevention Dec. 11 released a report that found last year’s version of the COVID-19 vaccine was 76% effective in preventing…
Headline
The AHA provided recommendations to the Food and Drug Administration Dec. 1 in response to a request for information on the measurement and evaluation of…
Headline
The Food and Drug Administration has identified a Class I recall of Baxter Life2000 Ventilation Systems due to a cybersecurity issue discovered through…
Headline
Flu cases are growing or likely growing in 39 states, according to the latest Centers for Disease Control and Prevention data from Nov. 11. COVID-19…
Headline
The Food and Drug Administration yesterday published an announcement from Otsuka ICU Medical saying that the company issued a voluntary recall for a mislabeled…

