FDA Issues Recommendations to Reduce Surgical Fires
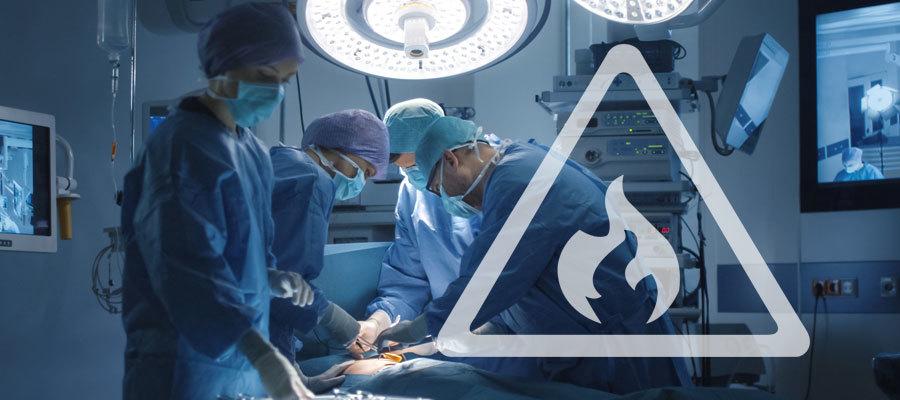
The Food and Drug Administration this week issued a safety communication reminding health care professionals of factors that increase the risk of surgical fires on or near a patient and offering recommendations to reduce these fires from occurring. Specific recommendations to reduce surgical fires include a fire risk assessment at the beginning of each surgical procedure; communication among surgical team members; safe use and administration of oxidizers, any devices that may serve as an ignition source and surgical suite items that may serve as a fuel source; and plan and practice how to manage a surgical fire. For more information, see the FDA safety communication.
Related News Articles
Headline
Thomas McGinn, M.D., senior executive vice president and chief physician executive officer at CommonSpirit Health, shares how the organization aligns…
Headline
Stephanie Calcasola, R.N., chief quality officer and vice president of quality and safety at Hartford HealthCare, unpacks the programs, technology and cultural…
Headline
Wendy Kim, DNP, R.N., vice president and chief nursing officer of Henry Ford Health in Michigan, shares how the system’s virtual nursing program is reducing…
Headline
The Centers for Medicare & Medicaid Services announced Dec. 30 that it will no longer require states to report measures reflecting the immunization status…
Perspective
If you had to describe a hospital’s mission, philosophy and guiding light with a single word, safety would sit at the top of the list.Providing a safe patient…
Headline
The AHA released a report Dec. 4 that found patient safety in hospitals and health systems across the nation continues to improve. The report, which uses data…

