ECRI: Surgical stapler misuse top health technology safety concern
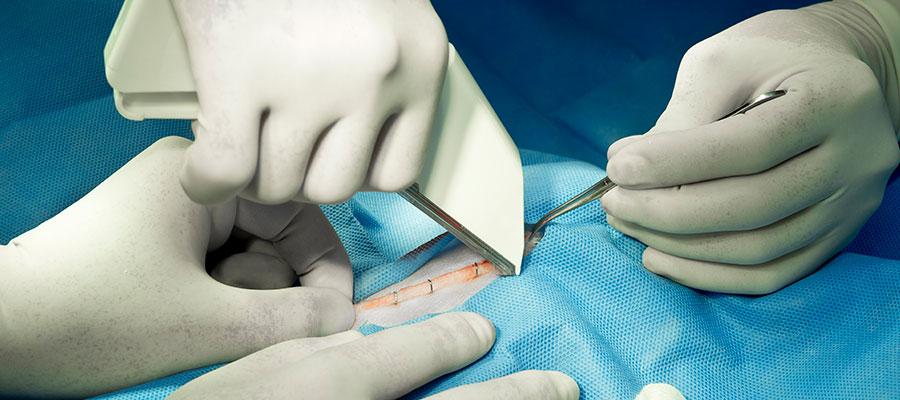
ECRI Institute today identified surgical stapler misuse as the leading health technology safety concern. In March, the Food and Drug Administration issued recommendations for health care providers to reduce the risk of adverse events associated with surgical staplers and staples for internal use. ECRI said other leading health technology risks include: point-of-care ultrasound; sterile processing in medical and dental offices; hemodialysis with central venous catheters; unproven surgical robotic procedures; alarm, alert and notification overload; cybersecurity in the connected home health care environment; missing patient implant data for magnetic resonance imaging; medication dose timing discrepancies in electronic health records; and loose medical device components.

