ASPR to test automated sterile saline system to help prevent IV shortages
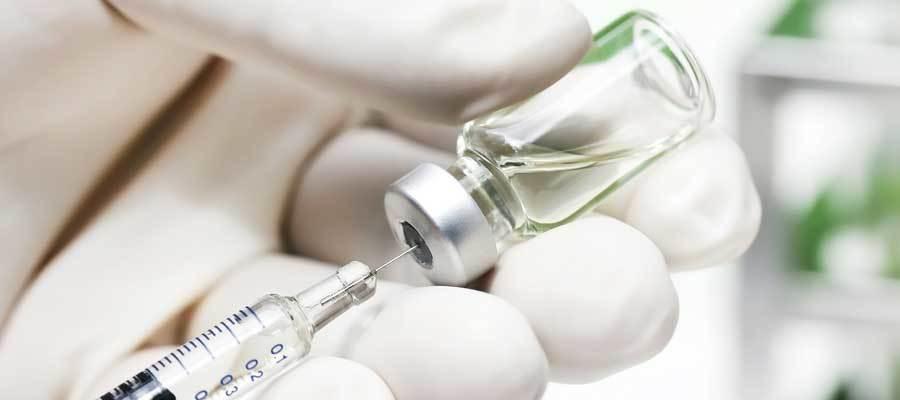
The Department of Health and Human Services’ Administration for Strategic Preparedness and Response June 5 announced it selected two locations to test an automated, point-of-care, sterile saline manufacturing system that could help prevent or mitigate intravenous fluid shortages in the U.S. The system, called Caspian, began development in 2019. One unit will be tested at Walter Reed Medical Center in Bethesda, Md., while the other will be tested and reviewed by the Food and Drug Administration. ASPR has been working with Manchester, N.H.-based DEKA Research and Development Corp. on manufacturing the units, with the intent of producing up to 500 bags of IV fluid per day.
Related News Articles
Headline
The Supreme Court Feb. 20 ruled that the International Emergency Economic Powers Act does not authorize the imposition of global tariffs. The court held that…
Headline
The Centers for Medicare & Medicaid Services Jan. 26 released an advanced notice of proposed rulemaking seeking comments on potential future policies to…
Headline
Olympus has expanded a voluntary recall of its ViziShot 2 FLEX Needles due to complaints of device components ejecting or attaching during…
Headline
The Trump administration announced a trade agreement with the U.K. Dec. 1 on pharmaceuticals that exempts U.K. drug products from Section 232 tariffs. In…
Headline
The AHA urged the Department of Commerce Oct. 17 to take a balanced approach to ensuring dependable and affordable access to personal protective equipment,…
Headline
The AHA’s Association for Health Care Resource & Materials Management has created a webpage featuring resources on tariffs impacting the health care supply…

