
Fact Sheet: Telehealth Waivers
November 2025
Background
At the outset of the COVID-19 pandemic, the federal government moved quickly to ensure hospitals and health systems were able to leverage telehealth services to respond efficiently and effectively to a wave of unprecedented need. These actions included the Centers for Medicare & Medicaid Services (CMS) waiving certain regulatory requirements and Congress providing significant legislative support to ensure hospitals and health systems could rapidly deploy virtual services.
AHA Take
The telehealth flexibilities granted resulted in significant benefits to patient care and are needed now more than ever to ensure patients’ continued access to high-quality care. Currently, there is a patchwork of temporary waivers for telehealth services that, barring further action, will expire on Jan. 30, 2026. If this occurs, we risk a telehealth “cliff” that would negatively impact patient access in all communities.
Recognizing both the immediate and potential long-term benefits of telehealth, we urge Congress, CMS and other executive agencies to take action to make critical telehealth flexibilities permanent.
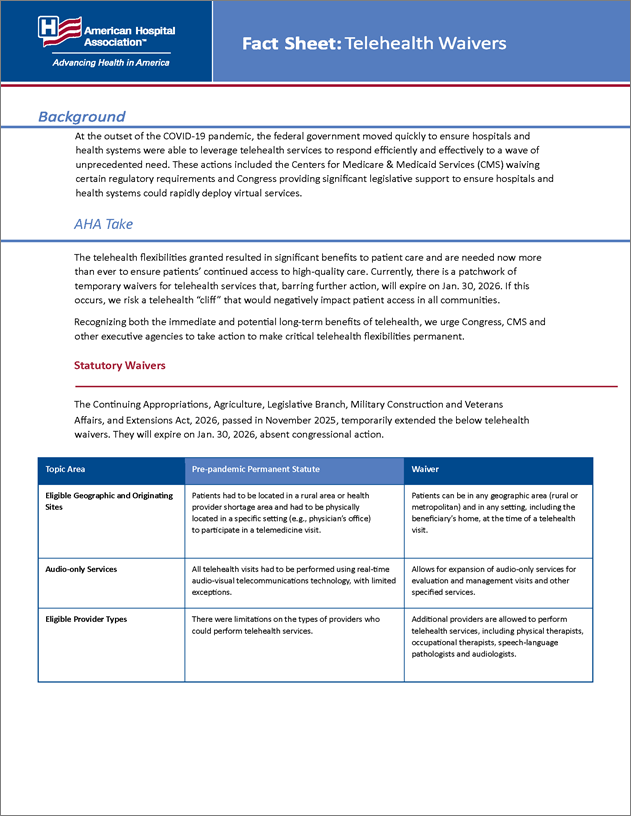
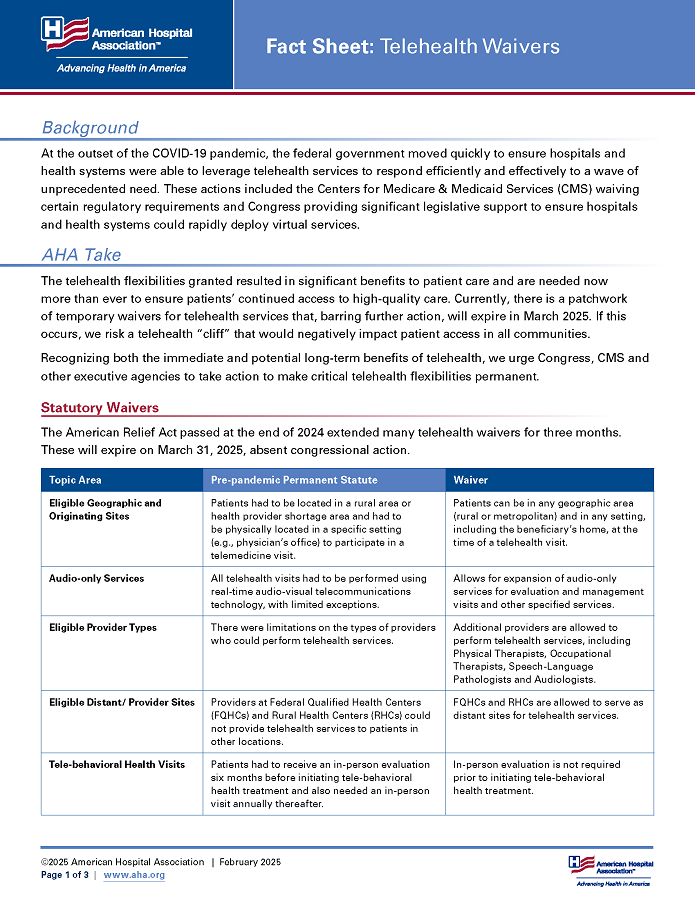
Statutory Waivers
The Continuing Appropriations, Agriculture, Legislative Branch, Military Construction and Veterans Affairs, and Extensions Act, 2026, passed in November 2025, temporarily extended the below telehealth waivers. They will expire on Jan. 30, 2026, absent congressional action.
| Topic Area | Pre-pandemic Permanent Statute | Waiver |
|---|---|---|
| Eligible Geographic and Originating Sites | Patients had to be located in a rural area or health provider shortage area and had to be physically located in a specific setting (e.g., physician’s office) to participate in a telemedicine visit. | Patients can be in any geographic area (rural or metropolitan) and in any setting, including the beneficiary’s home, at the time of a telehealth visit. |
| Audio-only Services | All telehealth visits had to be performed using real-time audio-visual telecommunications technology, with limited exceptions. | Allows for expansion of audio-only services for evaluation and management visits and other specified services. |
| Eligible Provider Types | There were limitations on the types of providers who could perform telehealth services. | Additional providers are allowed to perform telehealth services, including physical therapists, occupational therapists, speech-language pathologists and audiologists. |
| Eligible Distant Sites | Providers at Federally Qualified Health Centers (FQHCs) and Rural Health Clinics (RHCs) could not provide telehealth services to patients in other locations. | FQHCs and RHCs are allowed to serve as distant sites for telehealth services. |
| Tele-behavioral Health Visits | Patients had to receive an in-person evaluation six months before initiating tele-behavioral health treatment and also needed an in-person visit annually thereafter. | In-person evaluation is not required prior to and after initiating tele-behavioral health treatment. |
Regulatory Waivers
CMS extended one telehealth waiver through Dec. 31, 2026, declined to extend another waiver and made several others permanent.
| Topic Area | Pre-pandemic Permanent Regulation | Waiver |
|---|---|---|
| Frequency Limitations | Limitations on the number of subsequent inpatient visits, nursing facility visits and critical care consultations furnished through telehealth. | CMS permanently removed these frequency limitations. |
| Virtual Direct Supervision | Direct supervision of diagnostic tests, services furnished incident to a physician’s services, and pulmonary, cardiac and intensive cardiac rehabilitation services required immediate in-person availability of the supervising practitioner. | CMS permanently allows direct supervision through virtual presence using real-time audio/video technology. |
| Virtual Supervision of Residents in Teaching Settings | Teaching physicians could meet requirements for supervising key or critical portions of resident services through virtual presence instead of physically in person, but only for services furnished in residency training sites in non-Metropolitan Service Areas (non-MSAs). | CMS permanently allows virtual presence for resident telehealth services in all training sites (MSAs and non-MSAs). |
| FQHC/RHC | FQHCs and RHCs cannot bill for telehealth services. | FQHCs and RHCs are permanently allowed to bill for mental health visits furnished through telehealth and can bill for non-behavioral health telehealth services (including audio only) through Dec. 31, 2026. |
| Reporting of Provider Home Address | Providers who perform telehealth services from their home were required to report their home address on enrollment, billing and claims forms. | Providers may choose to suppress home address details from appearing publicly. |
The Drug Enforcement Administration (DEA) also issued temporary waivers regarding prescribing of controlled substances. Without action, these waivers will expire on Dec. 31, 2025.
| Topic Area | Pre-pandemic Permanent Regulation | Waiver |
|---|---|---|
| In-person Visit Requirements for Prescribing of Controlled Substances | Prior to prescribing controlled substances, the prescribing practitioner was required to conduct one in-person evaluation of the patient prior to prescribing. This could be waived through a special registration process per statute, but DEA has not finalized a regulation on what this special registration process would entail. | The in-person evaluation requirement is temporarily waived through Dec. 31, 2025. (Note: We recommend creation of a special registration process to waive the in-person visit requirement.) |
Take Action
The AHA urges Congress, CMS and the DEA to permanently adopt telehealth policies.
Other Resources
- Taking Action to Extend Telehealth and Hospital-at-home Programs | AHA News
- CMS Urged to Remove Telehealth Provider Home Address Reporting Requirements | AHA
- AHA Comments to CMS on CY 2026 Physician Fee Schedule Proposed Rule | AHA
- AHA Urges Congress to Make Telehealth Flexibilities Permanent | AHA News
- AHA Comments on 340B Drug Pricing Program, IRF Payments, Physician Fee Schedule and Telehealth | AHA
- AHA Letter of Support for Senate CONNECT Health Act of 2023 (S. 2016) | AHA
- AHA’s Feedback to the Senate Re: The CONNECT Act | AHA
- AHA Comments on the SUPPORT for Patients and Communities Reauthorization Act | AHA
- AHA Letter to DEA Regarding Request for Release of Special Registration for Telemedicine Regulation | AHA